Journal Publications
Samir Kumar Mondal, Sakshi Singh and Shantanu Pal
https://pubs.rsc.org/en/content/articlelanding/2025/ra/d5ra07899g


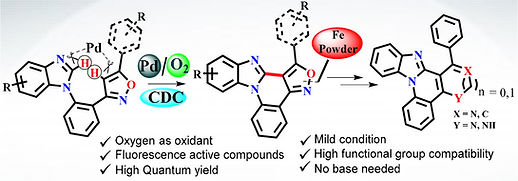
Sayan Mukherjee, Ujala Rani, Girish Chandra and Shantanu Pal
https://pubs.rsc.org/en/content/articlelanding/2025/ob/d5ob00997a

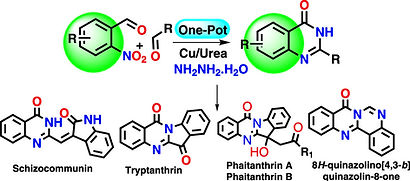
Samir Kumar Mondal, Shantanu Pal
https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/ejoc.202500203


Subrata Sahoo, Manthri Atchuta Rao and Shantanu Pal
https://pubs.acs.org/doi/full/10.1021/acs.joc.3c00766


Subrata Sahoo, Shantanu Pal
https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/slct.202300290



19. Novel homologated-apio adenosine derivatives as A 3 adenosine receptor agonists: design, synthesis and molecular docking studies
Amarendra Panda, Rayhan Gafur Biswas, Shantanu Pal
https://pubs.rsc.org/en/content/articlehtml/2016/ra/c5ra26416b
18. A common and versatile synthetic route to (−) and (+) pentenomycin I,(+) halopentenomycin I and dehydropentenomycin
Sulagna Das, Amarendra Panda, Shantanu Pal
https://www.sciencedirect.com/science/article/pii/S0008621515002256


17. Lead tetraacetate mediated one pot oxidative cleavage and acetylation reaction: an approach to apio and homologated apio pyrimidine nucleosides and their anticancer activity
Amarendra Panda, Sehbanul Islam, Manas Kumar Santra, Shantanu Pal
https://pubs.rsc.org/en/content/articlehtml/2015/ra/c5ra19080k

-
S. Pal, W. J. Cho, S. A. Choe, C. Heller, Z. G. Gao, M. Chinn, K. A. acobson, X. Hou, S. K. Lee, H. O. Kim, L. S. Jeong “Structure–activity relationships of truncated adenosine derivatives as highly potent and selective human A3 adenosine receptor antagonists” Bioorg. Med. Chem. 2009, 17, 3733-3738.
-
L. S. Jeong, S. Pal, S. A. Choe, W. J. Choi, K. A. Jacobson, Z. G. Gao, A. M. Klutz, X. Hou, H.O. Kim, H. W. Lee, S. K. Lee, D. K. Tosh, H. R. Moon “Structure–activity relationships of truncated D and L-4’-thioadenosine derivatives as highly potent and selective as human A3 adenosine receptor antagonists” J. Med. Chem. 2008, 51, 6609-6613.
-
X. Hou, S. Pal, W. J. Choi, H. O. Kim, A. Tipnis, K. A. Jacobson and L. S. Jeong. “Design and synthesis of truncated 4’-thioadenosine derivatives as potent and selective A3 adenosine receptor” Nucleic Acids Symposium Series 2008, 52, 641-642.
-
D. K. Tosh, W. J. Choi, H. O. Kim, Y. Lee, S. Pal, X. Hou, J. Choi, S. Choi, L. S. Jeong “Stereoselective synthesis and conformational study of novel 2’,3’-Didehydro-2’,3’-dideoxy-4’-selenonucleoside.” J. Org. Chem. 2008, 73, 4259-4262.
-
L. S. Jeong, H. W. Lee, H. O. Kim, D. K. Tosh, S. Pal; W. J. Choi, Z. G. Gao, A. R. Patel, W. Williams, K. A. Jacobson, H. D. Kim “Structure–activity relationships of 2-Chloro-N6-substituted-4′-thioadenosine-5′-N,N-dialkyluronamides as human A3 adenosine receptor antagonists” Biorg. Med. Chem. Lett. 2008, 18, 1612-1616.
-
L. S. Jeong, L. X. Zhao, W. J. Choi, S. Pal, Y. H. Park, S. K. Lee, M. W. Chum, Y. B. Lee, C. H. Anh, H. R. Moon “Synthesis and Antitumor activity of fluorocyclopentenyl-pyrimidines.” Nucleosides, Nucleotides Nucleic acids 2007, 26, 713-716.
-
M. W. Chum, H. W. Lee, J. H. Kim, H. O. Kim, K. Man, S. Pal; H. R. Moon, L. S. Jeong “Stereoselective synthesis of Homo-apioneplanocin A as potential inhibitor of S-adenosylhomocysteine hydrolase” Nucleosides, Nucleotides Nucleic acids, 2007, 26, 729-732.
-
T. Wang, H. J. Lee, D. K. Tosh, H. O. Kim, S. Pal, S. Choi, Y. Lee, H. R. Moon, L. X. Zhao, K. M. Lee, L. S. Jeong “Design, synthesis, and molecular modeling studies of 5′-deoxy-5′-ureidoadenosine: 5'-ureido group as multiple hydrogen bonding donors in the active site of S-adenosylhomocysteine hydrolase”. Biorg. Med. Chem. Lett., 2007, 17, 4456-4459.
-
V. Singh, S. Pal, D. K Tosh, S. M. Mobin “Molecular complexity from aromatics, cycloaddition of cyclohexa-2,4-dienones, sigmatropic 1,2-acyl shift and ring-closing metathesis: A new, efficient, and stereoselective synthesis of (±) hirsutic acid C and medium ring carbocycles” Tetrahedron, 2007, 63, 2446-2454.
-
V. Singh, S. Pal, S. M. Mobin “Cycloaddition between electron deficient -systems, photochemical and radical induced reactions: A novel, general and stereoselective route to polyfunctionalised bridge bicyclo[2.2.2]octanes, bicyclo[3.3.0]-, [4.2.0]octanes and tricycle[4.3.1.03,7]decanes.” J. Org. Chem., 2006, 71, 3014-3025.
-
V. Singh, S. I. Iyer, S. Pal “Recent approaches towards synthesis of cis-decalins”Tetrahedron 2005, 61, 9197-9231.
-
V. Singh, S. Lahiri, S. Pal. “Molecular complexity from aromatics: Synthesis and photoreaction of endo-tricyclo[5.2.2.02,6]undecane: A stereoselective route to tricyclic framework of protoilludanes” Biorg. Med. Chem. Lett., 2005, 15, 4367-4369.
-
V. Singh, S. Pal, S. M. Mobin “Cycloaddition of cyclohexa-2,4-dienones with electron deficient 2partners: A novel and stereoselective route to functionlised bicyclo[2.2.2]octenones”. Chem. Commum., 2002, 2050-2051.
Book Chapters
16. Highly regio- and diastereoselective, acidic clay supported intramolecular nitrile oxide–alkene cycloaddition on d-ribose derived nitriles: an efficient synthetic route to isoxazoline fused five and six membered carbocycles
Amarendra Panda, Sulagna Das, Shantanu Pal
https://www.sciencedirect.com/science/article/pii/S0008621514002456


-
L. S. Jeong, D. K. Tosh, S. Pal “Synthesis of fluoroneplanocin A” In Current protocols in nucleic acid chemistry ed. Beaucage, S. L. John Wiley & Sons: New York 2008, Chapter 14, unit 14.6.
-
D. K. Tosh, H. O. Kim, S. Pal, J. A. Lee and L. S. Jeong “Synthesis and biological activity of Neplanocin A and analogues” In Modified Nucleosides: in biochemistry, biotechnology and medicine ed. Herdewijn, P. John Wiley & Sons: New York 2008, Chapter 21, pp 525.
© 2017 Shantanu Pal Research Group